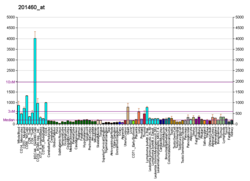
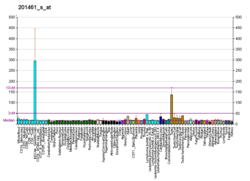
MAPKAPK2
| MAPKAPK2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MAPKAPK2, MAPKAP-K2, MK-2, MK2, mitogen-activated protein kinase-activated protein kinase 2, MAPK activated protein kinase 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 602006; MGI: 109298; HomoloGene: 56412; GeneCards: MAPKAPK2; OMA:MAPKAPK2 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
MAP kinase-activated protein kinase 2 is an enzyme that in humans is encoded by the MAPKAPK2 gene.[5][6][7]
Function
This gene encodes a member of the Ser/Thr protein kinase family. This kinase is regulated through direct phosphorylation by p38 MAP kinase. In conjunction with p38 MAP kinase, this kinase is known to be involved in many cellular processes including stress and inflammatory responses, nuclear export, gene expression regulation and cell proliferation. Heat shock protein HSP27 was shown to be its major direct substrate in vivo. Two transcript variants encoding two different isoforms have been found for this gene.[8]
Vascular barrier
MK2 pathway has been demonstrated to have a key role in maintaining and repairing the integrity of endothelial barrier in the lung via actin[9] and vimentin remodeling. Activation of MK2 via its phosphorylation by p38 has been shown to restore the vascular barrier[7] and repair vascular leak,[10] associated with over 60 medical conditions, including Acute Respiratory Distress Syndrome (ARDS), a major cause of death around the world.[11]
SASP initiation
MAPKAPK2 mediates the initiation of the senescence-associated secretory phenotype (SASP) by mTOR (mechanistic target of rapamycin).[12][13] Interleukin 1 alpha (IL1A) is found on the surface of senescent cells, where it contributes to the production of SASP factors due to a positive feedback loop with NF-κB.[14][15] Translation of mRNA for IL1A is highly dependent upon mTOR activity.[16] mTOR activity increases levels of IL1A, mediated by MAPKAPK2.[14]
See also
- SB 203580, suppresses the activation of MAPKAPK2
- MK2-AP directly activates MAPKAPK2 independent of p38.[7]
Interactions
MAPKAPK2 has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000162889 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000016528 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Zu YL, Wu F, Gilchrist A, et al. (April 1994). "The primary structure of a human MAP kinase activated protein kinase 2". Biochemical and Biophysical Research Communications. 200 (2): 1118–24. doi:10.1006/bbrc.1994.1566. PMID 8179591.
- ^ Stokoe D, Caudwell B, Cohen PT, et al. (December 1993). "The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2". The Biochemical Journal. 296 ( Pt 3) (Pt 3): 843–9. doi:10.1042/bj2960843. PMC 1137771. PMID 8280084.
- ^ a b c Liu T, Warburton RR, Hill NS, et al. (August 2015). "Anthrax lethal toxin-induced lung injury and treatment by activating MK2". Journal of Applied Physiology. 119 (4): 412–9. doi:10.1152/japplphysiol.00335.2015. PMC 4538279. PMID 26066827.
- ^ "Entrez Gene: MAPKAPK2 mitogen-activated protein kinase-activated protein kinase 2".
- ^ Sousa AM, Liu T, Guevara O, et al. (April 2007). "Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2". Journal of Cellular Biochemistry. 100 (6): 1581–92. doi:10.1002/jcb.21154. PMC 2586991. PMID 17163490.
- ^ Liu T, Milia E, Warburton RR, et al. (April 2012). "Anthrax lethal toxin disrupts the endothelial permeability barrier through blocking p38 signaling". Journal of Cellular Physiology. 227 (4): 1438–45. doi:10.1002/jcp.22859. PMC 4254851. PMID 21618534.
- ^ Pham T, Rubenfeld GD (April 2017). "Fifty Years of Research in ARDS. The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review". American Journal of Respiratory and Critical Care Medicine. 195 (7): 860–870. doi:10.1164/rccm.201609-1773CP. PMID 28157386. S2CID 23293950.
- ^ Yessenkyzy A, Saliev T, Zhanaliyeva M, et al. (May 2020). "Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research". Nutrients. 12 (5): 1344. doi:10.3390/nu12051344. PMC 7285205. PMID 32397145.
- ^ Papadopoli D, Boulay K, Kazak L, et al. (2019). "mTOR as a central regulator of lifespan and aging". F1000Research. 8: 998. doi:10.12688/f1000research.17196.1. PMC 6611156. PMID 31316753.
- ^ a b Laberge RM, Sun Y, Orjalo AV, et al. (August 2015). "MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation". Nature Cell Biology. 17 (8): 1049–61. doi:10.1038/ncb3195. PMC 4691706. PMID 26147250.
- ^ Wang R, Yu Z, Sunchu B, et al. (June 2017). "Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism". Aging Cell. 16 (3): 564–574. doi:10.1111/acel.12587. PMC 5418203. PMID 28371119.
- ^ Wang R, Sunchu B, Perez VI (August 2017). "Rapamycin and the inhibition of the secretory phenotype". Experimental Gerontology. 94: 89–92. doi:10.1016/j.exger.2017.01.026. PMID 28167236. S2CID 4960885.
- ^ a b Rane MJ, Coxon PY, Powell DW, et al. (February 2001). "p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils". The Journal of Biological Chemistry. 276 (5): 3517–23. doi:10.1074/jbc.M005953200. PMID 11042204.
- ^ Janknecht R (November 2001). "Cell type-specific inhibition of the ETS transcription factor ER81 by mitogen-activated protein kinase-activated protein kinase 2". The Journal of Biological Chemistry. 276 (45): 41856–61. doi:10.1074/jbc.M106630200. PMID 11551945.
- ^ a b Yannoni YM, Gaestel M, Lin LL (April 2004). "P66(ShcA) interacts with MAPKAP kinase 2 and regulates its activity". FEBS Letters. 564 (1–2): 205–11. doi:10.1016/S0014-5793(04)00351-5. PMID 15094067. S2CID 43606580.
- ^ Dondelinger Y, Delanghe T, Rojas-Rivera D, et al. (October 2017). "MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death". Nature Cell Biology. 19 (10): 1237–1247. doi:10.1038/ncb3608. PMID 28920952. S2CID 25779284.
Further reading
- Kapopara PR, von Felden J, Soehnlein O, et al. (December 2014). "Deficiency of MAPK-activated protein kinase 2 (MK2) prevents adverse remodelling and promotes endothelial healing after arterial injury". Thrombosis and Haemostasis. 112 (6): 1264–76. doi:10.1160/TH14-02-0174. PMID 25120198. S2CID 12322445.
- Ben-Levy R, Hooper S, Wilson R, et al. (September 1998). "Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2". Current Biology. 8 (19): 1049–57. Bibcode:1998CBio....8.1049B. doi:10.1016/S0960-9822(98)70442-7. PMID 9768359. S2CID 15627349.
- Stokoe D, Engel K, Campbell DG, et al. (November 1992). "Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins". FEBS Letters. 313 (3): 307–13. doi:10.1016/0014-5793(92)81216-9. PMID 1332886.
- Vulliet PR, Woodgett JR, Cohen P (November 1984). "Phosphorylation of tyrosine hydroxylase by calmodulin-dependent multiprotein kinase". The Journal of Biological Chemistry. 259 (22): 13680–3. doi:10.1016/S0021-9258(18)89798-8. PMID 6150037.
- Engel K, Schultz H, Martin F, et al. (November 1995). "Constitutive activation of mitogen-activated protein kinase-activated protein kinase 2 by mutation of phosphorylation sites and an A-helix motif". The Journal of Biological Chemistry. 270 (45): 27213–21. doi:10.1074/jbc.270.45.27213. PMID 7592979.
- Lavoie JN, Lambert H, Hickey E, et al. (January 1995). "Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27". Molecular and Cellular Biology. 15 (1): 505–16. doi:10.1128/MCB.15.1.505. PMC 232001. PMID 7799959.
- Sutherland C, Alterio J, Campbell DG, et al. (October 1993). "Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2". European Journal of Biochemistry. 217 (2): 715–22. doi:10.1111/j.1432-1033.1993.tb18297.x. PMID 7901013.
- Knauf U, Jakob U, Engel K, et al. (January 1994). "Stress- and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance". The EMBO Journal. 13 (1): 54–60. doi:10.1002/j.1460-2075.1994.tb06234.x. PMC 394778. PMID 7905823.
- Freshney NW, Rawlinson L, Guesdon F, et al. (September 1994). "Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27". Cell. 78 (6): 1039–49. doi:10.1016/0092-8674(94)90278-X. PMID 7923354. S2CID 37608621.
- Rivera VM, Miranti CK, Misra RP, et al. (October 1993). "A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity". Molecular and Cellular Biology. 13 (10): 6260–73. doi:10.1128/mcb.13.10.6260. PMC 364685. PMID 8413226.
- Beyaert R, Cuenda A, Vanden Berghe W, et al. (April 1996). "The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor". The EMBO Journal. 15 (8): 1914–23. doi:10.1002/j.1460-2075.1996.tb00542.x. PMC 450110. PMID 8617238.
- Ben-Levy R, Leighton IA, Doza YN, et al. (December 1995). "Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2". The EMBO Journal. 14 (23): 5920–30. doi:10.1002/j.1460-2075.1995.tb00280.x. PMC 394711. PMID 8846784.
- Tan Y, Rouse J, Zhang A, et al. (September 1996). "FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2". The EMBO Journal. 15 (17): 4629–42. doi:10.1002/j.1460-2075.1996.tb00840.x. PMC 452194. PMID 8887554.
- Huang CK, Zhan L, Ai Y, et al. (January 1997). "LSP1 is the major substrate for mitogen-activated protein kinase-activated protein kinase 2 in human neutrophils". The Journal of Biological Chemistry. 272 (1): 17–9. doi:10.1074/jbc.272.1.17. PMID 8995217.
- Krump E, Sanghera JS, Pelech SL, et al. (January 1997). "Chemotactic peptide N-formyl-met-leu-phe activation of p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils". The Journal of Biological Chemistry. 272 (2): 937–44. doi:10.1074/jbc.272.2.937. PMID 8995385.
- Engel K, Kotlyarov A, Gaestel M (June 1998). "Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation". The EMBO Journal. 17 (12): 3363–71. doi:10.1093/emboj/17.12.3363. PMC 1170674. PMID 9628873.
- Craxton A, Shu G, Graves JD, et al. (October 1998). "p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes". Journal of Immunology. 161 (7): 3225–36. doi:10.4049/jimmunol.161.7.3225. PMID 9759836. S2CID 39342790.
- Heidenreich O, Neininger A, Schratt G, et al. (May 1999). "MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo". The Journal of Biological Chemistry. 274 (20): 14434–43. doi:10.1074/jbc.274.20.14434. PMID 10318869.
- v
- t
- e
-
 1kwp: Crystal Structure of MAPKAP2
1kwp: Crystal Structure of MAPKAP2 -
 1nxk: Crystal structure of staurosporine bound to MAP KAP kinase 2
1nxk: Crystal structure of staurosporine bound to MAP KAP kinase 2 -
 1ny3: Crystal structure of ADP bound to MAP KAP kinase 2
1ny3: Crystal structure of ADP bound to MAP KAP kinase 2 -
 2jbo: PROTEIN KINASE MK2 IN COMPLEX WITH AN INHIBITOR (CRYSTAL FORM-1, SOAKING)
2jbo: PROTEIN KINASE MK2 IN COMPLEX WITH AN INHIBITOR (CRYSTAL FORM-1, SOAKING) -
 2jbp: PROTEIN KINASE MK2 IN COMPLEX WITH AN INHIBITOR (CRYSTAL FORM-2, CO-CRYSTALLIZATION)
2jbp: PROTEIN KINASE MK2 IN COMPLEX WITH AN INHIBITOR (CRYSTAL FORM-2, CO-CRYSTALLIZATION) -
 2onl: Crystal Structure of the p38a-MAPKAP kinase 2 Heterodimer
2onl: Crystal Structure of the p38a-MAPKAP kinase 2 Heterodimer -
 2oza: Structure of p38alpha complex
2oza: Structure of p38alpha complex
 | This article on a gene on human chromosome 1 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e