COPG
Protein-coding gene in humans
| COPG1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | COPG1, COPG, coatomer protein complex subunit gamma 1, COPI coat complex subunit gamma 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 615525; MGI: 1858696; HomoloGene: 56745; GeneCards: COPG1; OMA:COPG1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
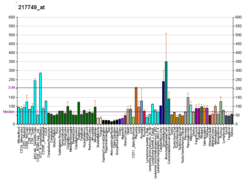
Coatomer subunit gamma is a protein that in humans is encoded by the COPG gene.[5][6] It is one of seven proteins in the COPI coatomer complex that coats vesicles as they bud from the Golgi complex.[5]
Interactions
COPG has been shown to interact with Dopamine receptor D1,[7] COPZ1[5][8] and COPB1.[5][9]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000181789 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000030058 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d Futatsumori M, Kasai K, Takatsu H, Shin HW, Nakayama K (February 2001). "Identification and characterization of novel isoforms of COP I subunits". J Biochem. 128 (5): 793–801. doi:10.1093/oxfordjournals.jbchem.a022817. PMID 11056392.
- ^ "Entrez Gene: COPG coatomer protein complex, subunit gamma".
- ^ Bermak, Jason C; Li Ming; Bullock Clayton; Weingarten Paul; Zhou Qun-Yong (February 2002). "Interaction of gamma-COP with a transport motif in the D1 receptor C-terminus". Eur. J. Cell Biol. 81 (2). Germany: 77–85. doi:10.1078/0171-9335-00222. ISSN 0171-9335. PMID 11893085.
- ^ Faulstich, D; Auerbach S; Orci L; Ravazzola M; Wegchingel S; Lottspeich F; Stenbeck G; Harter C; Wieland F T; Tschochner H (October 1996). "Architecture of coatomer: molecular characterization of delta-COP and protein interactions within the complex" (PDF). J. Cell Biol. 135 (1). UNITED STATES: 53–61. doi:10.1083/jcb.135.1.53. ISSN 0021-9525. PMC 2121028. PMID 8858162.
- ^ Lowe, M; Kreis T E (November 1996). "In vivo assembly of coatomer, the COP-I coat precursor". J. Biol. Chem. 271 (48). UNITED STATES: 30725–30. doi:10.1074/jbc.271.48.30725. ISSN 0021-9258. PMID 8940050.
External links
- Human COPG1 genome location and COPG1 gene details page in the UCSC Genome Browser.
Further reading
- Lowe M, Kreis TE (1997). "In vivo assembly of coatomer, the COP-I coat precursor". J. Biol. Chem. 271 (48): 30725–30. doi:10.1074/jbc.271.48.30725. PMID 8940050.
- Pavel J, Harter C, Wieland FT (1998). "Reversible dissociation of coatomer: functional characterization of a beta/delta-coat protein subcomplex". Proc. Natl. Acad. Sci. U.S.A. 95 (5): 2140–5. Bibcode:1998PNAS...95.2140P. doi:10.1073/pnas.95.5.2140. PMC 19276. PMID 9482852.
- Fischer KD, Helms JB, Zhao L, Wieland FT (2000). "Site-specific photocrosslinking to probe interactions of Arf1 with proteins involved in budding of COPI vesicles". Methods. 20 (4): 455–64. doi:10.1006/meth.2000.0958. PMID 10720466.
- Eugster A, Frigerio G, Dale M, Duden R (2000). "COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP". EMBO J. 19 (15): 3905–17. doi:10.1093/emboj/19.15.3905. PMC 306616. PMID 10921873.
- Hu RM, Han ZG, Song HD, et al. (2000). "Gene expression profiling in the human hypothalamus-pituitary-adrenal axis and full-length cDNA cloning". Proc. Natl. Acad. Sci. U.S.A. 97 (17): 9543–8. Bibcode:2000PNAS...97.9543H. doi:10.1073/pnas.160270997. PMC 16901. PMID 10931946.
- Sullivan BM, Harrison-Lavoie KJ, Marshansky V, et al. (2000). "RGS4 and RGS2 bind coatomer and inhibit COPI association with Golgi membranes and intracellular transport". Mol. Biol. Cell. 11 (9): 3155–68. doi:10.1091/mbc.11.9.3155. PMC 14982. PMID 10982407.
- Hahn Y, Lee YJ, Yun JH, et al. (2000). "Duplication of genes encoding non-clathrin coat protein gamma-COP in vertebrate, insect and plant evolution". FEBS Lett. 482 (1–2): 31–6. doi:10.1016/S0014-5793(00)02033-0. PMID 11018518. S2CID 36356269.
- Paulsson KM, Kleijmeer MJ, Griffith J, et al. (2002). "Association of tapasin and COPI provides a mechanism for the retrograde transport of major histocompatibility complex (MHC) class I molecules from the Golgi complex to the endoplasmic reticulum". J. Biol. Chem. 277 (21): 18266–71. doi:10.1074/jbc.M201388200. PMID 11884415.
- Bermak JC, Li M, Bullock C, et al. (2002). "Interaction of gamma-COP with a transport motif in the D1 receptor C-terminus". Eur. J. Cell Biol. 81 (2): 77–85. doi:10.1078/0171-9335-00222. PMID 11893085.
- Kanzaki M, Watson RT, Hou JC, et al. (2003). "Small GTP-binding protein TC10 differentially regulates two distinct populations of filamentous actin in 3T3L1 adipocytes". Mol. Biol. Cell. 13 (7): 2334–46. doi:10.1091/mbc.01-10-0490. PMC 117317. PMID 12134073.
- Xu Y, Martin S, James DE, Hong W (2003). "GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus". Mol. Biol. Cell. 13 (10): 3493–507. doi:10.1091/mbc.E02-01-0004. PMC 129961. PMID 12388752.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Rohde HM, Cheong FY, Konrad G, et al. (2004). "The human phosphatidylinositol phosphatase SAC1 interacts with the coatomer I complex". J. Biol. Chem. 278 (52): 52689–99. doi:10.1074/jbc.M307983200. PMID 14527956.
- Watson PJ, Frigerio G, Collins BM, et al. (2004). "Gamma-COP appendage domain - structure and function". Traffic. 5 (2): 79–88. doi:10.1111/j.1600-0854.2004.00158.x. PMID 14690497. S2CID 24249308.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Ewing RM, Chu P, Elisma F, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- v
- t
- e
PDB gallery
-
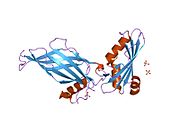 1pzd: Structural Identification of a conserved appendage domain in the carboxyl-terminus of the COPI gamma-subunit.
1pzd: Structural Identification of a conserved appendage domain in the carboxyl-terminus of the COPI gamma-subunit. -
 1r4x: Crystal Structure Analys of the Gamma-COPI Appendage domain
1r4x: Crystal Structure Analys of the Gamma-COPI Appendage domain
 | This article on a gene on human chromosome 3 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e