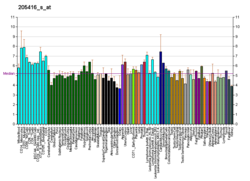
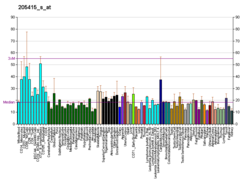
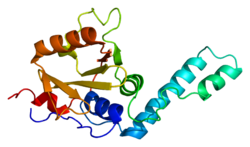
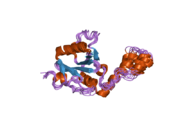Ataxin 3
| ATXN3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ATXN3, AT3, ATX3, JOS, MJD, MJD1, SCA3, Ataxin 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 607047; MGI: 1099442; HomoloGene: 3658; GeneCards: ATXN3; OMA:ATXN3 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Ataxin-3 is a protein that in humans is encoded by the ATXN3 gene.[5][6]
Clinical significance
Machado–Joseph disease, also known as spinocerebellar ataxia-3, is an autosomal dominant neurologic disorder. The protein encoded by the ATXN3 gene contains CAG repeats in the coding region, and the expansion of these repeats from the normal 13-36 to 68-79 is the cause of Machado–Joseph disease. This disorder is thus a trinucleotide repeat disorder type I known as a polyglutamine (PolyQ) disease. There is an inverse correlation between the age of onset and CAG repeat numbers. Alternatively spliced transcript variants encoding different isoforms have been described for this gene.[6]
Interactions
Ataxin 3 has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000066427 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021189 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Takiyama Y, Nishizawa M, Tanaka H, Kawashima S, Sakamoto H, Karube Y, Shimazaki H, Soutome M, Endo K, Ohta S (Jul 1993). "The gene for Machado-Joseph disease maps to human chromosome 14q". Nature Genetics. 4 (3): 300–4. doi:10.1038/ng0793-300. PMID 8358439. S2CID 27424416.
- ^ a b "Entrez Gene: ATXN3 ataxin 3".
- ^ a b Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N (Jul 2000). "Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B". Human Molecular Genetics. 9 (12): 1795–803. doi:10.1093/hmg/9.12.1795. PMID 10915768.
- ^ Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K (Sep 2003). "Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis". Molecular and Cellular Biology. 23 (18): 6469–83. doi:10.1128/MCB.23.18.6469-6483.2003. PMC 193705. PMID 12944474.
- ^ Wang Q, Li L, Ye Y (Mar 2008). "Inhibition of p97-dependent protein degradation by Eeyarestatin I". The Journal of Biological Chemistry. 283 (12): 7445–54. doi:10.1074/jbc.M708347200. PMC 2276333. PMID 18199748.
Further reading
- Goto J, Watanabe M, Ichikawa Y, Yee SB, Ihara N, Endo K, Igarashi S, Takiyama Y, Gaspar C, Maciel P, Tsuji S, Rouleau GA, Kanazawa I (Aug 1997). "Machado-Joseph disease gene products carrying different carboxyl termini". Neuroscience Research. 28 (4): 373–7. doi:10.1016/S0168-0102(97)00056-4. PMID 9274833. S2CID 27241785.
- Schöls L, Vieira-Saecker AM, Schöls S, Przuntek H, Epplen JT, Riess O (Jun 1995). "Trinucleotide expansion within the MJD1 gene presents clinically as spinocerebellar ataxia and occurs most frequently in German SCA patients". Human Molecular Genetics. 4 (6): 1001–5. doi:10.1093/hmg/4.6.1001. PMID 7655453.
- Stevanin G, Cancel G, Dürr A, Chneiweiss H, Dubourg O, Weissenbach J, Cann HM, Agid Y, Brice A (Jan 1995). "The gene for spinal cerebellar ataxia 3 (SCA3) is located in a region of approximately 3 cM on chromosome 14q24.3-q32.2". American Journal of Human Genetics. 56 (1): 193–201. PMC 1801316. PMID 7825578.
- Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I (Nov 1994). "CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1". Nature Genetics. 8 (3): 221–8. doi:10.1038/ng1194-221. PMID 7874163. S2CID 29188685.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Ikeda H, Yamaguchi M, Sugai S, Aze Y, Narumiya S, Kakizuka A (Jun 1996). "Expanded polyglutamine in the Machado-Joseph disease protein induces cell death in vitro and in vivo". Nature Genetics. 13 (2): 196–202. doi:10.1038/ng0696-196. PMID 8640226. S2CID 21966287.
- Paulson HL, Das SS, Crino PB, Perez MK, Patel SC, Gotsdiner D, Fischbeck KH, Pittman RN (Apr 1997). "Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain". Annals of Neurology. 41 (4): 453–62. doi:10.1002/ana.410410408. PMID 9124802. S2CID 43460483.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thornberry N, Pinsky L, Kakizuka A, Ross CA, Nicholson DW, Bredesen DE, Hayden MR (Apr 1998). "Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract". The Journal of Biological Chemistry. 273 (15): 9158–67. doi:10.1074/jbc.273.15.9158. PMID 9535906.
- Tait D, Riccio M, Sittler A, Scherzinger E, Santi S, Ognibene A, Maraldi NM, Lehrach H, Wanker EE (Jun 1998). "Ataxin-3 is transported into the nucleus and associates with the nuclear matrix". Human Molecular Genetics. 7 (6): 991–7. CiteSeerX 10.1.1.588.3970. doi:10.1093/hmg/7.6.991. PMID 9580663.
- Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N (Jul 2000). "Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B". Human Molecular Genetics. 9 (12): 1795–803. doi:10.1093/hmg/9.12.1795. PMID 10915768.
- Ichikawa Y, Goto J, Hattori M, Toyoda A, Ishii K, Jeong SY, Hashida H, Masuda N, Ogata K, Kasai F, Hirai M, Maciel P, Rouleau GA, Sakaki Y, Kanazawa I (2001). "The genomic structure and expression of MJD, the Machado-Joseph disease gene". Journal of Human Genetics. 46 (7): 413–22. doi:10.1007/s100380170060. PMID 11450850.
- Chai Y, Shao J, Miller VM, Williams A, Paulson HL (Jul 2002). "Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis". Proceedings of the National Academy of Sciences of the United States of America. 99 (14): 9310–5. Bibcode:2002PNAS...99.9310C. doi:10.1073/pnas.152101299. PMC 123137. PMID 12084819.
- Yoshida H, Yoshizawa T, Shibasaki F, Shoji S, Kanazawa I (Jul 2002). "Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch". Neurobiology of Disease. 10 (2): 88–99. doi:10.1006/nbdi.2002.0502. PMID 12127147. S2CID 25183307.
- Li F, Macfarlan T, Pittman RN, Chakravarti D (Nov 2002). "Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities". The Journal of Biological Chemistry. 277 (47): 45004–12. doi:10.1074/jbc.M205259200. PMID 12297501.
- Albrecht M, Hoffmann D, Evert BO, Schmitt I, Wüllner U, Lengauer T (Feb 2003). "Structural modeling of ataxin-3 reveals distant homology to adaptins". Proteins. 50 (2): 355–70. doi:10.1002/prot.10280. PMID 12486728. S2CID 25660172.
- Marchal S, Shehi E, Harricane MC, Fusi P, Heitz F, Tortora P, Lange R (Aug 2003). "Structural instability and fibrillar aggregation of non-expanded human ataxin-3 revealed under high pressure and temperature". The Journal of Biological Chemistry. 278 (34): 31554–63. doi:10.1074/jbc.M304205200. PMID 12766160.
External links
- GeneReviews/NCBI/NIH/UW entry on Spinocerebellar Ataxia Type 3
- Human ATXN3 genome location and ATXN3 gene details page in the UCSC Genome Browser.
- v
- t
- e
-
 1yzb: Solution structure of the Josephin domain of Ataxin-3
1yzb: Solution structure of the Josephin domain of Ataxin-3 -
 2aga: De-ubiquitinating function of ataxin-3: insights from the solution structure of the Josephin domain
2aga: De-ubiquitinating function of ataxin-3: insights from the solution structure of the Josephin domain -
 2dos: Structural basis for the recognition of Lys48-linked polyubiquitin chain by the Josephin domain of ataxin-3, a putative deubiquitinating enzyme
2dos: Structural basis for the recognition of Lys48-linked polyubiquitin chain by the Josephin domain of ataxin-3, a putative deubiquitinating enzyme