Ammonium hexafluoroaluminate
 | |
| Names | |
|---|---|
| Other names Ammonium aluminium fluoride | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.029.138 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
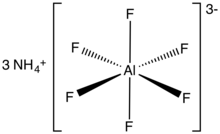
Chemical formula | (NH4)3[AlF6] |
| Molar mass | 195.09 g/mol |
| Appearance | White crystalline powder |
| Density | 1.78 g/cm3 at 20 °C |
| Melting point | 126.1 °C (259.0 °F; 399.2 K) |
| Boiling point | 239.5 °C (463.1 °F; 512.6 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant (Xi) |
| GHS labelling: | |
Pictograms |  |
| Danger | |
Hazard statements | H301, H311, H330, H331 |
Precautionary statements | P260, P261, P264, P270, P271, P280, P284, P301+P310, P302+P352, P304+P340, P310, P311, P312, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |  2 0 0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Ammonium hexafluoroaluminate is an inorganic compound with the chemical formula of (NH4)3[AlF6]. It is a white solid. Upon heating, it converts to aluminium trifluoride, a reaction that releases hydrogen fluoride.[1] It has also been used as a precursor to zeolites.[2]
Preparation
Ammonium hexafluoroaluminate can be obtained by the reaction of ammonium fluoride and aluminium hydroxide.[3]
References
- ^ Alonso, C.; Morato, A.; Medina, F.; Guirado, F.; Cesteros, Y.; Salagre, P.; Sueiras, J. E.; Terrado, R.; Giralt, A. (2000). "Preparation and Characterization of Different Phases of Aluminum Trifluoride". Chemistry of Materials. 12 (4): 1148–1155. doi:10.1021/cm991195g.
- ^ Kao, Hsien-Ming; Ting, Chun-Chiang; Chao, Shih-Wei (2005). "Post-synthesis alumination of mesoporous silica SBA-15 with high framework aluminum content using ammonium hexafluoroaluminate". Journal of Molecular Catalysis A: Chemical. 235 (1–2): 200–208. doi:10.1016/j.molcata.2005.03.026.
- ^ hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1975). Handbuch der präparativen anorganischen Chemie / 1 (in German). Stuttgart: Enke. p. 239. ISBN 3-432-02328-6. OCLC 310719485.
- v
- t
- e
Ammonium salts
| monatomic anions |
|
|---|---|
| oxyanions |
|
| other anions |
|
- Aluminon
- Ammonium acetate
- Ammonium adipate
- Ammonium benzoate
- Ammonium bituminosulfonate
- Ammonium carbamate
- Ammonium citrate
- Ammonium diethyl dithiophosphate
- Ammonium ferric citrate
- Ammonium formate
- Ammonium fumarate
- Ammonium glutamate
- Ammonium lactate
- Ammonium lauryl sulfate
- Ammonium malate
- Ammonium nonanoate
- Ammonium oxalate
- Ammonium picrate
- Ammonium perfluorononanoate
- Ammonium propionate
- Ammonium thioglycolate
- Cupferron
- Ferric ammonium oxalate
- Murexide
![{\displaystyle \mathrm {6\ NH_{4}F+Al(OH)_{3}\longrightarrow (NH_{4})_{3}[AlF_{6}]+3\ NH_{4}OH} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/3a8bc07d0a5a46dc2ccb13d129f84fb5279c14fc)












