Ammonium oxalate
 | |
| Names | |
|---|---|
| IUPAC name Ammonium oxalate | |
| Systematic IUPAC name Ammonium ethanedioate | |
| Other names Diammonium oxalate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.012.912 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
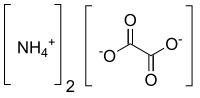
Chemical formula | [NH4]2C2O4 |
| Molar mass | 124.096 g·mol−1 |
| Appearance | Colorless or white crystalline solid |
| Density | 1.5 g/cm3[1] |
| Melting point | 70 C (158 F, 343.15 K) |
Solubility in water | 5.20 g/(100 ml) (25 °C)[1] |
| Hazards | |
| GHS labelling: | |
Hazard statements | H302, H312, H319 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Ammonium oxalate is a chemical compound with the chemical formula [NH4]2C2O4. Its formula is often written as (NH4)2C2O4 or (COONH4)2. It is an ammonium salt of oxalic acid. It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4). The structure of ammonium oxalate is ([NH4]+)2[C2O4]2−. Ammonium oxalate sometimes comes as a monohydrate ([NH4]2C2O4·H2O). It is a colorless or white salt under standard conditions and is odorless and non-volatile. It occurs in many plants and vegetables.
Vertebrate
It is produced in the body of vertebrates by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine.[2] It is a constituent of some types of kidney stone.[3][4] It is also found in guano.
Mineralogy
Oxammite is a natural mineral form of ammonium oxalate. This mineral is extremely rare. It is an organic mineral derived from guano.[5]
Chemistry
Ammonium oxalate is used as an analytical reagent and general reducing agent.[2] It and other oxalates are used as anticoagulants, to preserve blood outside the body.[citation needed]
Earth sciences
Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron(II)-bearing minerals (such as magnetite) and organic matter.[6][page needed]
References
- ^ a b John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–41. ISBN 978-1138561632.
- ^ a b National Center for Biotechnology Information. PubChem Compound Database; CID 14213 (accessed 15 November 2016).
- ^ The International Pharmacopoeia, p.1292, Volume 1, World Health Organization, 2006 ISBN 92-4-156301-X.
- ^ N G Coley, "The collateral sciences in the work of Golding Bird (1814–1854)", Medical History, iss.4, vol.13, October 1969, pp.372.
- ^ "Home". mindat.org.
- ^ Rayment, George; Lyons, David (2011). Soil Chemical Methods - Australasia. CSIRO Publishing. ISBN 9780643101364.
- v
- t
- e
| monatomic anions |
|
|---|---|
| oxyanions |
|
| other anions |
|
- Aluminon
- Ammonium acetate
- Ammonium adipate
- Ammonium benzoate
- Ammonium bituminosulfonate
- Ammonium carbamate
- Ammonium citrate
- Ammonium diethyl dithiophosphate
- Ammonium ferric citrate
- Ammonium formate
- Ammonium fumarate
- Ammonium glutamate
- Ammonium lactate
- Ammonium lauryl sulfate
- Ammonium malate
- Ammonium nonanoate
- Ammonium oxalate
- Ammonium picrate
- Ammonium perfluorononanoate
- Ammonium propionate
- Ammonium thioglycolate
- Cupferron
- Ferric ammonium oxalate
- Murexide









