Vanadocene
 | |
| Names | |
|---|---|
| IUPAC name Bis(cyclopentadienyl)vanadium | |
| Other names Vanadocene | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ECHA InfoCard | 100.149.756 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
Chemical formula | V(C5H5)2 |
| Molar mass | 181.128 g/mol |
| Appearance | Violet Crystal |
| Melting point | 167 °C (333 °F; 440 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Vanadocene, bis(η5-cyclopentadienyl) vanadium, is the organometallic compound with the formula V(C5H5)2, commonly abbreviated Cp2V. It is a violet crystalline, paramagnetic solid. Vanadocene has relatively limited practical use, but it has been extensively studied.
Structure and bonding
V(C5H5)2 is a metallocene, a class of organometallic compounds that typically have a metal ion sandwiched between two cyclopentadienyl rings. In the solid state, the molecule has D5d symmetry. The vanadium(II) center resides equidistant between the center of the two cyclopentadienyl rings at a crystallographic center of inversion. The average V-C bond distance is 226 pm.[1] The Cp rings of vanadocene are dynamically disordered at temperatures above 170 K and are only fully ordered at 108 K.
Preparation
Vanadocene was first prepared in 1954 by Birmingham, Fischer, and Wilkinson via a reduction of vanadocene dichloride with aluminum hydride, after which vanadocene was sublimed in vacuum at 100 ˚C.[2] A modern synthesis of vanadocene that allows production in higher quantities requires treating [V2Cl3(THF)6]2[Zn2Cl6] with cyclopentadienylsodium.[3]
- 2 [V2Cl3(THF)6]2[Zn2Cl6] + 8 NaCp + THF → 4 Cp2V
Properties
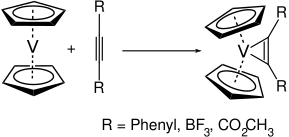
Vanadocene is a reactive molecule. As it only has 15 valence electrons available, it readily reacts with many ligands. With alkynes, for example, it reacts to yield the corresponding vanadium-cyclopropene complexes.[4]
One reaction involves carbon monoxide, leading to an ionic vanadocene derivative when performed in inert atmosphere:
- Cp2V + V(CO)6 → [Cp2V(CO)2][V(CO)6]
Vanadocene is readily oxidized to the monocation when treated with a ferrocenium salt in toluene.[5]
- VCp2 + [FeCp2]BR4 → [VCp2]BR4 + FeCp2 (R = Ph or 4-C6H4F)
These monocations are extremely air-sensitive and have a redox potential of -1.10 V.[5]
Vanadocene reacts with high pressures of carbon monoxide to give CpV(CO)4.[6]
References
- ^ Robin D. Rogers; Jerry L. Atwood; Don Foust & Marvin D. Rausch (1981). "Crystal Structure of Vanadocene". Journal of Crystal and Molecular Structure. 11 (5–6): 183–188. doi:10.1007/BF01210393. S2CID 93048446.
- ^ Birmingham, J. M., A. K. Fischer, and G. Wilkinson (1955). "The Reduction of Bis-cyclopentadienyl Compounds". Naturwissenschaften. 42 (4): 96. Bibcode:1955NW.....42Q..96B. doi:10.1007/BF00617242. S2CID 44523847.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lorber, C. "Vanadium Organometallics." Chapter 5.01. Comprehensive Organometallic Chemistry III. Elsevier, 2007. 1-60.
- ^ Jordan, Markus (2009). Azine in der Koordinationssphäre von Vanadocenderivaten unterschiedlicher Oxidationsstufen (PhD thesis). Universität Oldenburg.
- ^ a b Calderazzo, Fausto, Isabella Ferri, Guido Pampaloni, and Ulli Englert (1999). "Oxidation Products of Vanadocene and of Its Permethylated Analogue, Including the Isolation and the Reactivity of the Unsolvated [VCp]Cation". Organometallics. 18 (13): 2452–2458. doi:10.1021/om9809320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ King, R.B.; Stone, F.G.A. (1963). "Cyclopentadienyl Metal Carbonyls and Some Derivatives". Inorganic Syntheses. Vol. 7. pp. 99–115. doi:10.1002/9780470132388.ch31. ISBN 9780470132388.
{{cite book}}:|journal=ignored (help)
- v
- t
- e
- V(CO)6
- VF2
- VBr2
- VCl2
- VI2
- VO
- VS
- VSO4
- VBr3
- VCl3
- VF3
- VI3
- VN
- V2O3
- V2(SO4)3
- V2S3
| Organovanadium(III) compounds |
|---|
- VC
- VO2
- VOCl2
- V(S2)2
- VCl4
- VF4
| Organovanadium(IV) compounds | |
|---|---|
| Vanadyl(IV) compounds |
|
- V2O5
- VOCl3
- VOF3
- VO2F
- VF5
- VCl5
- NH4VO3
- VOPO4
- VO+2
| Vanadyl(V) compounds |
|
|---|