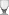Tetrasodium EDTA
 | |
| Names | |
|---|---|
| IUPAC name Tetrasodium N,N′-(ethane-1,2-diyl)bis[N-(carboxylatomethyl)glycinate] | |
| Systematic IUPAC name Tetrasodium 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetate | |
| Other names E39 Trilon B | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.000.522 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C10H12N2Na4O8 |
| Molar mass | 380.171 g·mol−1 |
| Appearance | White solid |
| Hazards | |
| GHS labelling: | |
Pictograms |   |
| Danger | |
Hazard statements | H302, H318 |
Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P310, P330, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Tetrasodium EDTA is the salt resulting from the neutralization of ethylenediaminetetraacetic acid with four equivalents of sodium hydroxide (or an equivalent sodium base). It is a white solid that is highly soluble in water. Commercial samples are often hydrated, e.g. Na4EDTA.4H2O. The properties of solutions produced from the anhydrous and hydrated forms are the same, provided they are at the same pH.
It is used as a source of the chelating agent EDTA4-. A 1% aqueous solution has a pH of approximately 11.3. When dissolved in neutral water, it converts partially to H2EDTA2-. Ethylenediaminetetraacetic acid is produced commercially via the intermediacy of tetrasodium EDTA.[1]
Products
The substance is also known as Dissolvine E-39. It is a salt of edetic acid. It has been known at least since 1954.[2][3] It is sometimes used as a chelating agent.[4][5][6][7]
The assignee on 5% of patents at the USPTO containing the substance is the firm Procter and Gamble. It is used most notably in cosmetics and hair and skin care products.
The substance has been used to aid in formulation of a removal product for rust, corrosion, and scale from ferrous metal, copper, brass, and other surfaces.[8]
At a concentration of 6%, it is the main active ingredient in some types of engine coolant system flushes.[9]
References
- ^ Hart, J. Roger. "Ethylenediaminetetraacetic Acid and Related Chelating Agents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_095. ISBN 978-3527306732.
- ^ "Tetrasodium ethylenediaminetetraacetate". European Chemicals Agency. Retrieved 3 April 2021.
- ^ T. T. Trujillo, H. Foreman (1954). "The metabolism of C14 labeled ethylenediaminetetraacetic acid in human beings". The Journal of Laboratory and Clinical Medicine. 43 (4): 566–571. PMID 13163555.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ Alessandro Fulgenzi; Maria Elena Ferrero (February 2019). "EDTA Chelation Therapy for the Treatment of Neurotoxicity". International Journal of Molecular Sciences. 20 (5): 1019. doi:10.3390/ijms20051019. PMC 6429616. PMID 30813622.
- ^ Swaran J.S. Flora; Vidhu Pachauri (June 2010). "Chelation in Metal Intoxication". International Journal of Environmental Research and Public Health. 7 (7): 2745–2788. doi:10.3390/ijerph7072745. PMC 2922724. PMID 20717537.
- ^ Zaitoun, Mohammed A.; Lin, C. T. (1997). "Chelating Behavior between Metal Ions and EDTA in Sol−Gel Matrix". The Journal of Physical Chemistry B. 101 (10): 1857–1860. doi:10.1021/jp963102d.
- ^ Thomas, Glenn C. Sr. (1995-11-21). "Rust, corrosion, and scale remover". US5468303A. United States Patent and Trademark Office.
- ^ "SAFETY DATA SHEET" (PDF). Fcsdchemicalsandlubricants.com. Retrieved 14 March 2022.
- v
- t
- e
system
| Alcohol intoxication | |
|---|---|
| Barbiturate overdose | |
| Benzodiazepine overdose | |
| GHB overdose | |
| Nerve agent / Organophosphate poisoning |
|
| Opioid overdose | |
| Reversal of neuromuscular blockade |
system
| Beta blocker | |
|---|---|
| Digoxin toxicity | |
| Anticoagulants |
|
| Arsenic poisoning | |
|---|---|
| Cyanide poisoning |
|
| Hydrofluoric acid | |
| Methanol / Ethylene glycol poisoning |
|
| Paracetamol toxicity (Acetaminophen) | |
| Toxic metals (cadmium | |
| Other |
- Copper sulfate
- Ipecacuanha
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III