Protein-coding gene in the species Homo sapiens
| RGS14 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2JNU, 2OM2, 2XNS, 3ONW, 3QI2 |
|
|
| Identifiers |
|---|
| Aliases | RGS14, regulator of G protein signaling 14 |
|---|
| External IDs | OMIM: 602513 MGI: 1859709 HomoloGene: 4735 GeneCards: RGS14 |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 5 (human)[1] |
|---|
| | Band | 5q35.3 | Start | 177,357,924 bp[1] |
|---|
| End | 177,372,596 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 13 (mouse)[2] |
|---|
| | Band | 13 B1|13 29.8 cM | Start | 55,517,545 bp[2] |
|---|
| End | 55,532,500 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - caudate nucleus
- putamen
- nucleus accumbens
- right lobe of liver
- blood
- monocyte
- spleen
- right uterine tube
- lymph node
- gallbladder
|
| | Top expressed in | - entorhinal cortex
- thymus
- nucleus accumbens
- hippocampus proper
- olfactory tubercle
- dentate gyrus
- blood
- Region I of hippocampus proper
- visual cortex
- superior frontal gyrus
|
| | More reference expression data |
|
|---|
| BioGPS | 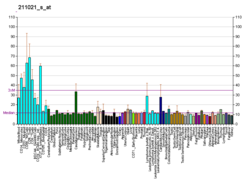
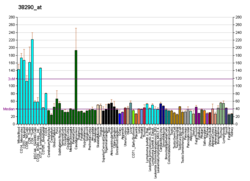
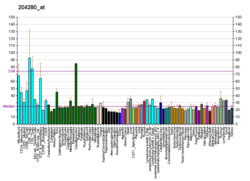 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - GTPase activating protein binding
- GTPase activator activity
- GTPase regulator activity
- microtubule binding
- protein binding
- GDP-dissociation inhibitor activity
- G-protein alpha-subunit binding
- signaling receptor complex adaptor activity
- protein kinase binding
- GTPase activity
| | Cellular component | - cytoplasm
- PML body
- centrosome
- postsynaptic membrane
- cell projection
- spindle pole
- nuclear body
- membrane
- postsynaptic density
- plasma membrane
- spindle
- dendritic spine
- synapse
- microtubule organizing center
- cell junction
- dendrite
- microtubule
- cytoskeleton
- nucleus
- glutamatergic synapse
| | Biological process | - visual learning
- intracellular signal transduction
- regulation of G protein-coupled receptor signaling pathway
- chromosome segregation
- learning
- response to oxidative stress
- cell division
- positive regulation of neurogenesis
- positive regulation of GTPase activity
- spindle organization
- long-term memory
- negative regulation of MAP kinase activity
- nucleocytoplasmic transport
- zygote asymmetric cell division
- negative regulation of ERK1 and ERK2 cascade
- cell cycle
- platelet-derived growth factor receptor signaling pathway
- negative regulation of signal transduction
- regulation of DNA-templated transcription in response to stress
- negative regulation of synaptic plasticity
- signal transduction
- long-term potentiation
- mitotic cell cycle
- negative regulation of G protein-coupled receptor signaling pathway
- modulation of chemical synaptic transmission
- G protein-coupled receptor signaling pathway
- positive regulation of signal transduction
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_006480
NM_001366617
NM_001366618 |
| |
|---|
| RefSeq (protein) | |
|---|
NP_006471
NP_001353546
NP_001353547 |
| |
|---|
| Location (UCSC) | Chr 5: 177.36 – 177.37 Mb | Chr 13: 55.52 – 55.53 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Regulator of G-protein signaling 14 (RGS14) is a protein that in humans is encoded by the RGS14 gene.[5]
Function
RGS14 is a member of the regulator of G protein signalling family. This protein contains one RGS domain, two Raf-like Ras-binding domains (RBDs), and one GoLoco motif. The protein attenuates the signaling activity of G-proteins by binding, through its GoLoco domain, to specific types of activated, GTP-bound G alpha subunits. Acting as a GTPase activating protein (GAP), the protein increases the rate of conversion of the GTP to GDP. This hydrolysis allows the G alpha subunits to bind G beta/gamma subunit heterodimers, forming inactive G-protein heterotrimers, thereby terminating the signal. Alternate transcriptional splice variants of this gene have been observed but have not been thoroughly characterized.[5]
Increasing the expression of the RGS14 protein in the V2 secondary visual cortex of mice promotes the conversion of short-term to long-term object-recognition memory.[6] Conversely RGS14 is enriched in CA2 pyramidal neurons and suppresses synaptic plasticity of these synapses and hippocampal-based learning and memory.[7]
Interactions
RGS14 has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000169220 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000052087 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: RGS14 regulator of G-protein signalling 14".
- ^ López-Aranda MF, López-Téllez JF, Navarro-Lobato I, Masmudi-Martín M, Gutiérrez A, Khan ZU (July 2009). "Role of layer 6 of V2 visual cortex in object-recognition memory". Science. 325 (5936): 87–9. Bibcode:2009Sci...325...87L. doi:10.1126/science.1170869. PMID 19574389. S2CID 23990759.
- Annalee Newitz (July 2, 2009). "A Drug That Could Give You Perfect Visual Memory". io9.
- ^ Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, Smith Y, Sweatt JD, Weinshenker D, Ressler KJ, Dudek SM, Hepler JR (September 2010). "RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory". Proc Natl Acad Sci U S A. 107 (39): 16994–8. Bibcode:2010PNAS..10716994L. doi:10.1073/pnas.1005362107. PMC 2947872. PMID 20837545.
- "Gene limits learning and memory in mice". MedicalDaily. 18 September 2010. Archived from the original on 2010-09-20.
- ^ a b Kimple RJ, De Vries L, Tronchère H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP (August 2001). "RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity". J. Biol. Chem. 276 (31): 29275–81. doi:10.1074/jbc.M103208200. PMID 11387333.
- ^ Hollinger S, Taylor JB, Goldman EH, Hepler JR (December 2001). "RGS14 is a bifunctional regulator of Galphai/o activity that exists in multiple populations in brain". J. Neurochem. 79 (5): 941–9. doi:10.1046/j.1471-4159.2001.00629.x. PMID 11739605. S2CID 22913974.
- ^ Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP (April 2002). "Structural determinants for GoLoco-induced inhibition of nucleotide release by Galpha subunits". Nature. 416 (6883): 878–81. Bibcode:2002Natur.416..878K. doi:10.1038/416878a. PMID 11976690. S2CID 4406208.
- ^ Shu FJ, Ramineni S, Amyot W, Hepler JR (January 2007). "Selective interactions between Gi alpha1 and Gi alpha3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization". Cell. Signal. 19 (1): 163–76. doi:10.1016/j.cellsig.2006.06.002. PMID 16870394.
Further reading
- Snow BE, Antonio L, Suggs S, et al. (1997). "Molecular cloning and expression analysis of rat Rgs12 and Rgs14". Biochem. Biophys. Res. Commun. 233 (3): 770–7. doi:10.1006/bbrc.1997.6537. PMID 9168931.
- Traver S, Bidot C, Spassky N, et al. (2001). "RGS14 is a novel Rap effector that preferentially regulates the GTPase activity of galphao". Biochem. J. 350 (1): 19–29. doi:10.1042/0264-6021:3500019. PMC 1221220. PMID 10926822.
- Cho H, Kozasa T, Takekoshi K, et al. (2000). "RGS14, a GTPase-activating protein for Gialpha, attenuates Gialpha- and G13alpha-mediated signaling pathways". Mol. Pharmacol. 58 (3): 569–76. doi:10.1124/mol.58.3.569. PMID 10953050.
- Hollinger S, Taylor JB, Goldman EH, Hepler JR (2001). "RGS14 is a bifunctional regulator of Gi/oalpha activity that exists in multiple populations in brain". Journal of Neurochemistry. 79 (5): 941–49. doi:10.1046/j.1471-4159.2001.00629.x. PMID 11739605. S2CID 22913974.
- Kimple RJ, De Vries L, Tronchère H, et al. (2001). "RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity". J. Biol. Chem. 276 (31): 29275–81. doi:10.1074/jbc.M103208200. PMID 11387333.
- Sierra DA, Gilbert DJ, Householder D, et al. (2002). "Evolution of the regulators of G-protein signaling multigene family in mouse and human". Genomics. 79 (2): 177–85. doi:10.1006/geno.2002.6693. PMID 11829488. S2CID 16065132.
- Kimple RJ, Kimple ME, Betts L, et al. (2002). "Structural determinants for GoLoco-induced inhibition of nucleotide release by Galpha subunits". Nature. 416 (6883): 878–81. Bibcode:2002Natur.416..878K. doi:10.1038/416878a. PMID 11976690. S2CID 4406208.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Hollinger S, Ramineni S, Hepler JR (2003). "Phosphorylation of RGS14 by protein kinase A potentiates its activity toward G alpha i.". Biochemistry. 42 (3): 811–9. doi:10.1021/bi026664y. PMID 12534294.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Martin-McCaffrey L, Willard FS, Oliveira-dos-Santos AJ, et al. (2004). "RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote". Dev. Cell. 7 (5): 763–9. doi:10.1016/j.devcel.2004.10.004. PMID 15525537.
- Martin-McCaffrey L, Willard FS, Pajak A, et al. (2006). "RGS14 is a microtubule-associated protein". Cell Cycle. 4 (7): 953–60. doi:10.4161/cc.4.7.1787. PMID 15917656. S2CID 37573929.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Shu FJ, Ramineni S, Amyot W, Hepler JR (2007). "Selective interactions between Gialpha1 and Gialpha3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization". Cellular Signalling. 19 (1): 941–49. doi:10.1016/j.cellsig.2006.06.002. PMID 16870394.
- Shu FJ, Ramineni S, Hepler JR (2009). "RGS14 is multifunctional scaffold that integrates G protein and Ras/Raf MAPkinase signaling pathways". Cellular Signalling: in press.
External links
- Overview of all the structural information available in the PDB for UniProt: O43566 (Regulator of G-protein signaling 14) at the PDBe-KB.
 | This article on a gene on human chromosome 5 is a stub. You can help Wikipedia by expanding it. |
![1kjy: Crystal Structure of Human G[alpha]i1 Bound to the GoLoco Motif of RGS14](//upload.wikimedia.org/wikipedia/commons/thumb/e/ea/PDB_1kjy_EBI.jpg/180px-PDB_1kjy_EBI.jpg) 1kjy: Crystal Structure of Human G[alpha]i1 Bound to the GoLoco Motif of RGS14
1kjy: Crystal Structure of Human G[alpha]i1 Bound to the GoLoco Motif of RGS14 2jnu: Solution structure of the RGS domain of human RGS14
2jnu: Solution structure of the RGS domain of human RGS14









![1kjy: Crystal Structure of Human G[alpha]i1 Bound to the GoLoco Motif of RGS14](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ea/PDB_1kjy_EBI.jpg/180px-PDB_1kjy_EBI.jpg)













