| GLRX3 |
|---|
|
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2DIY, 2WZ9, 2YAN, 3ZYW |
|
|
| Identifiers |
|---|
| Aliases | GLRX3, GLRX4, GRX3, GRX4, PICOT, TXNL2, TXNL3, glutaredoxin 3 |
|---|
| External IDs | OMIM: 612754; MGI: 1353653; HomoloGene: 4769; GeneCards: GLRX3; OMA:GLRX3 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 10 (human)[1] |
|---|
| | Band | 10q26.3 | Start | 130,136,391 bp[1] |
|---|
| End | 130,184,521 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 7 (mouse)[2] |
|---|
| | Band | 7|7 F4 | Start | 137,039,343 bp[2] |
|---|
| End | 137,070,323 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - buccal mucosa cell
- islet of Langerhans
- right testis
- left testis
- mucosa of esophagus
- right adrenal gland
- right adrenal cortex
- left adrenal gland
- left adrenal cortex
- body of pancreas
|
| | Top expressed in | - spermatid
- spermatocyte
- yolk sac
- ankle joint
- corneal stroma
- plantaris muscle
- entorhinal cortex
- extensor digitorum longus muscle
- morula
- superior frontal gyrus
|
| | More reference expression data |
|
|---|
| BioGPS | 

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein-disulfide reductase activity
- electron transfer activity
- iron-sulfur cluster binding
- protein binding
- metal ion binding
- protein kinase C binding
- RNA binding
- identical protein binding
- glutathione disulfide oxidoreductase activity
| | Cellular component | - cytoplasm
- dendrite
- cell cortex
- Z disc
- extracellular exosome
- nucleus
- cytosol
| | Biological process | - cell redox homeostasis
- negative regulation of cardiac muscle hypertrophy
- regulation of the force of heart contraction
- [2Fe-2S cluster assembly]
- protein maturation by iron-sulfur cluster transfer
- electron transport chain
- cellular iron ion homeostasis
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001199868
NM_006541
NM_001321980 |
| |
|---|
| RefSeq (protein) | |
|---|
NP_001186797
NP_001308909
NP_006532 |
| |
|---|
| Location (UCSC) | Chr 10: 130.14 – 130.18 Mb | Chr 7: 137.04 – 137.07 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
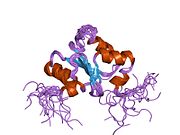 1wik: Solution Structure of the PICOT homology 2 domain of the mouse PKC-interacting cousin of thioredoxin protein
1wik: Solution Structure of the PICOT homology 2 domain of the mouse PKC-interacting cousin of thioredoxin protein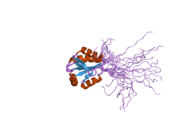 2diy: The solution structure of the thioredoxin domain of human Thioredoxin-like protein 2
2diy: The solution structure of the thioredoxin domain of human Thioredoxin-like protein 2






















