ARF6
| ARF6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ARF6, ADP-ribosylation factor 6, ADP ribosylation factor 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 600464 MGI: 99435 HomoloGene: 1256 GeneCards: ARF6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
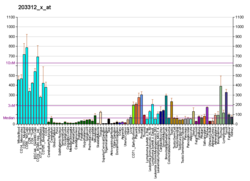
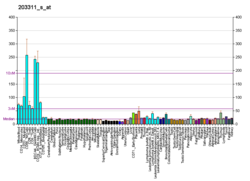
ADP-ribosylation factor 6 (ARF6) is a member of the ADP ribosylation factor family of GTP-binding proteins. ARF6 has a variety of cellular functions that are frequently involved in trafficking of biological membranes and transmembrane protein cargo. ARF6 has specifically been implicated in endocytosis of plasma membrane proteins and also, to a lesser extent, plasma membrane protein recycling.
Function
This gene encodes a member of the human ARF gene family, which is part of the Ras superfamily. The ARF genes encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking and as activators of phospholipase D. The product of this gene is localized to the plasma membrane, and regulates vesicular trafficking, remodelling of membrane lipids, and signaling pathways that lead to actin remodeling. A pseudogene of this gene is located on chromosome 7.[5]
ARF6 can interact with βarrestin upon receptor activation.
Interactions
ARF6 has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000165527 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000044147 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: ARF6 ADP-ribosylation factor 6".
- ^ Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ (November 2001). "beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis". J. Biol. Chem. 276 (45): 42509–13. doi:10.1074/jbc.M108399200. PMID 11533043.
- ^ D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L (September 1997). "A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements". EMBO J. 16 (17): 5445–54. doi:10.1093/emboj/16.17.5445. PMC 1170175. PMID 9312003.
- ^ Shin OH, Exton JH (August 2001). "Differential binding of arfaptin 2/POR1 to ADP-ribosylation factors and Rac1". Biochem. Biophys. Res. Commun. 285 (5): 1267–73. doi:10.1006/bbrc.2001.5330. PMID 11478794.
- ^ Mitchell R, Robertson DN, Holland PJ, Collins D, Lutz EM, Johnson MS (September 2003). "ADP-ribosylation factor-dependent phospholipase D activation by the M3 muscarinic receptor". J. Biol. Chem. 278 (36): 33818–30. doi:10.1074/jbc.M305825200. PMID 12799371.
- ^ Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rossé C, Camonis J, Chavrier P (December 2003). "ARF6 controls post-endocytic recycling through its downstream exocyst complex effector". J. Cell Biol. 163 (5): 1111–21. doi:10.1083/jcb.200305029. PMC 2173613. PMID 14662749.
- ^ Prieto C, De Las Rivas J (July 2006). "APID: Agile Protein Interaction DataAnalyzer". Nucleic Acids Res. 34 (Web Server issue): W298–302. doi:10.1093/nar/gkl128. PMC 1538863. PMID 16845013. Archived from the original on 2010-04-09.
- ^ Peru y Colón de Portugal RL, Acevedo SF, Rodan AR, Chang LY, Eaton BA, Rothenfluh A (December 2012). "Adult neuronal Arf6 controls ethanol-induced behavior with Arfaptin downstream of Rac1 and RhoGAP18B". J. Neurosci. 32 (49): 17706–13. doi:10.1523/JNEUROSCI.1944-12.2012. PMC 3535434. PMID 23223291.
Further reading
- Sabe H (2004). "Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion". J. Biochem. 134 (4): 485–9. doi:10.1093/jb/mvg181. PMID 14607973.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Tsuchiya M, Price SR, Tsai SC, Moss J, Vaughan M (1991). "Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells". J. Biol. Chem. 266 (5): 2772–7. doi:10.1016/S0021-9258(18)49913-9. PMID 1993656.
- Stearns T, Willingham MC, Botstein D, Kahn RA (1990). "ADP-ribosylation factor is functionally and physically associated with the Golgi complex". Proc. Natl. Acad. Sci. U.S.A. 87 (3): 1238–42. Bibcode:1990PNAS...87.1238S. doi:10.1073/pnas.87.3.1238. PMC 53446. PMID 2105501.
- D'Souza-Schorey C, Stahl PD (1995). "Myristoylation is required for the intracellular localization and endocytic function of ARF6". Exp. Cell Res. 221 (1): 153–9. doi:10.1006/excr.1995.1362. PMID 7589240.
- D'Souza-Schorey C, Li G, Colombo MI, Stahl PD (1995). "A regulatory role for ARF6 in receptor-mediated endocytosis". Science. 267 (5201): 1175–8. Bibcode:1995Sci...267.1175D. doi:10.1126/science.7855600. PMID 7855600. S2CID 23249341.
- Welsh CF, Moss J, Vaughan M (1995). "ADP-ribosylation factors: a family of approximately 20-kDa guanine nucleotide-binding proteins that activate cholera toxin". Mol. Cell. Biochem. 138 (1–2): 157–66. doi:10.1007/BF00928458. PMID 7898460. S2CID 42819002.
- Amor JC, Harrison DH, Kahn RA, Ringe D (1995). "Structure of the human ADP-ribosylation factor 1 complexed with GDP". Nature. 372 (6507): 704–8. Bibcode:1994Natur.372..704C. doi:10.1038/372704a0. PMID 7990966. S2CID 4362056.
- Orcl L, Palmer DJ, Amherdt M, Rothman JE (1993). "Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol". Nature. 364 (6439): 732–4. Bibcode:1993Natur.364..732O. doi:10.1038/364732a0. PMID 8355790. S2CID 4348442.
- Helms JB, Palmer DJ, Rothman JE (1993). "Two distinct populations of ARF bound to Golgi membranes". J. Cell Biol. 121 (4): 751–60. doi:10.1083/jcb.121.4.751. PMC 2119793. PMID 8491770.
- Cavenagh MM, Whitney JA, Carroll K, Zhang Cj, Boman AL, Rosenwald AG, Mellman I, Kahn RA (1996). "Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane". J. Biol. Chem. 271 (36): 21767–74. doi:10.1074/jbc.271.36.21767. PMID 8702973.
- D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L (1997). "A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements". EMBO J. 16 (17): 5445–54. doi:10.1093/emboj/16.17.5445. PMC 1170175. PMID 9312003.
- Frank S, Upender S, Hansen SH, Casanova JE (1998). "ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6". J. Biol. Chem. 273 (1): 23–7. doi:10.1074/jbc.273.1.23. PMID 9417041.
- Yang CZ, Heimberg H, D'Souza-Schorey C, Mueckler MM, Stahl PD (1998). "Subcellular distribution and differential expression of endogenous ADP-ribosylation factor 6 in mammalian cells". J. Biol. Chem. 273 (7): 4006–11. doi:10.1074/jbc.273.7.4006. PMID 9461590.
- Mao M, Fu G, Wu JS, Zhang QH, Zhou J, Kan LX, Huang QH, He KL, Gu BW, Han ZG, Shen Y, Gu J, Yu YP, Xu SH, Wang YX, Chen SJ, Chen Z (1998). "Identification of genes expressed in human CD34(+) hematopoietic stem/progenitor cells by expressed sequence tags and efficient full-length cDNA cloning". Proc. Natl. Acad. Sci. U.S.A. 95 (14): 8175–80. Bibcode:1998PNAS...95.8175M. doi:10.1073/pnas.95.14.8175. PMC 20949. PMID 9653160.
- Andreev J, Simon JP, Sabatini DD, Kam J, Plowman G, Randazzo PA, Schlessinger J (1999). "Identification of a new Pyk2 target protein with Arf-GAP activity". Mol. Cell. Biol. 19 (3): 2338–50. doi:10.1128/MCB.19.3.2338. PMC 84026. PMID 10022920.
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG (1999). "ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements". J. Cell Sci. 112 (6): 855–66. doi:10.1242/jcs.112.6.855. PMID 10036235.
- Kim HS (1999). "Assignment of the human ADP-ribosylation factor 6 (ARF6) gene to chromosome 7q22.1 by radiation hybrid mapping". Cytogenet. Cell Genet. 84 (1–2): 94. doi:10.1159/000015225. PMID 10343114. S2CID 31452287.
- Langille SE, Patki V, Klarlund JK, Buxton JM, Holik JJ, Chawla A, Corvera S, Czech MP (1999). "ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1". J. Biol. Chem. 274 (38): 27099–104. doi:10.1074/jbc.274.38.27099. PMID 10480924.
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y (1999). "Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation". Cell. 99 (5): 521–32. doi:10.1016/S0092-8674(00)81540-8. PMID 10589680.
- Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW (2005). "Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis". Circ. Res. 96 (4): 467–75. doi:10.1161/01.RES.0000158286.51045.16. PMID 15692085.
External links
- ARF6+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human ARF6 genome location and ARF6 gene details page in the UCSC Genome Browser.
- v
- t
- e
-
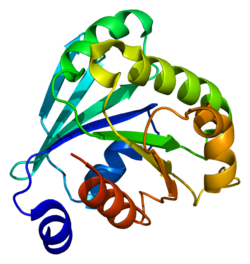
 1e0s: SMALL G PROTEIN ARF6-GDP
1e0s: SMALL G PROTEIN ARF6-GDP -
 2a5d: Structural basis for the activation of cholera toxin by human ARF6-GTP
2a5d: Structural basis for the activation of cholera toxin by human ARF6-GTP -
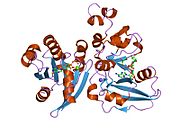 2a5f: Cholera toxin A1 subunit bound to its substrate, NAD+, and its human protein activator, ARF6
2a5f: Cholera toxin A1 subunit bound to its substrate, NAD+, and its human protein activator, ARF6 -
 2a5g: Cholera toxin A1 subunit bound to ARF6(Q67L)
2a5g: Cholera toxin A1 subunit bound to ARF6(Q67L) -
 2j5x: STRUCTURE OF THE SMALL G PROTEIN ARF6 IN COMPLEX WITH GTPGAMMAS
2j5x: STRUCTURE OF THE SMALL G PROTEIN ARF6 IN COMPLEX WITH GTPGAMMAS